In recent times, major spice brands from India faced regulatory issues across many countries globally.
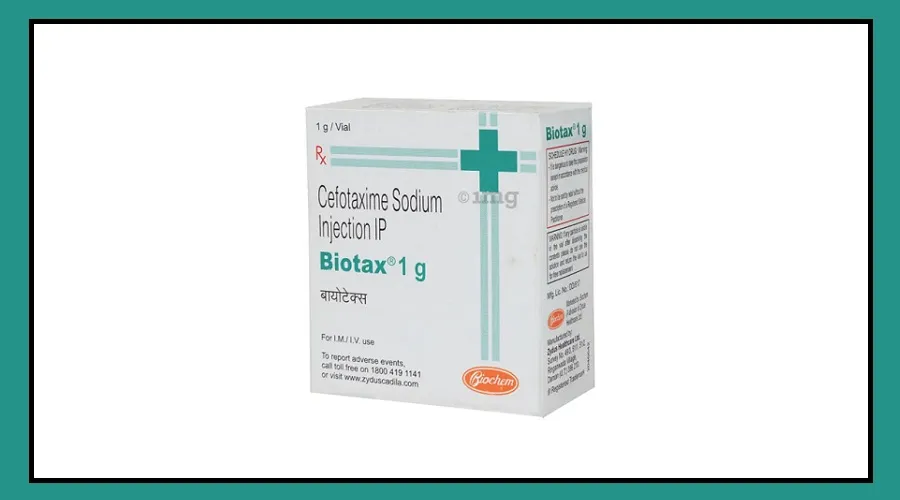
This scrutiny has also extended to Indian medicines, with Nepal banning Zydus Lifesciences’ Biotax 1gm.
The Department of Drug Administration in Nepal stated that the injection did not meet production standards and failed lab tests.
Consequently, the sale and distribution of this injection are now banned in Nepal. Zydus Lifesciences, however, maintains that its injection is entirely safe.
The batch of injections failed the lab test
The Department of Drug Administration has expressed health concerns regarding the Biotax 1GM injection in a report by The Kathmandu Post.
They found that batch F300460 of this injection failed lab tests and could pose risks to patients.
Consequently, the manufacturing company, importers, and distributors have been instructed to halt its sale and distribution immediately.
The injection is produced by Zydus Healthcare Limited, based in Ahmedabad.
There will be no shortage of medicines, many products are available
The Department of Drug Administration’s spokesperson, Pramod KC, stated that their investigation revealed the injection isn’t safe.
Biotax 1GM is typically used to treat infections in various parts of the body like the brain, lungs, ears, skin, soft tissues, bones, blood, and heart, including during surgeries.
However, there are alternative injections in the market, so its absence won’t impact treatment availability.
Zydus Lifesciences denies all allegations
In contrast, Zydus Lifesciences, an Indian pharmaceutical company, has refuted these accusations.
The company informed Business Standard that the report was misleading and inaccurate, asserting the safety of their product.
Regarding the concerns raised by DDA about the sterile water quantity in the injection, the company spokesperson clarified it as a misunderstanding.
They emphasized their track record of selling quality medicines in Nepal since 2018.